$SGMO Liver-directed gene therapy for Wilson's disease: http://www.ncbi.nlm.nih.gov/pubmed/26409215
Tuesday, September 29, 2015
Monday, September 28, 2015
BioMarin Enrolls First Patient in Phase 1/2 Trial of Gene Therapy Drug Candidate BMN 270 for the Treatment of Hemophilia A
BMN 270 is the First AAV-Factor VIII Gene Therapy to be Investigated in a Clinical Trial Setting for Patients With Hemophilia A
SAN RAFAEL, Calif., Sept. 28, 2015 (GLOBE NEWSWIRE) -- BioMarin Pharmaceutical Inc. (NASDAQ:BMRN) announced today that it has enrolled the first patient in a Phase 1/2 trial for BMN 270, an investigational gene therapy for the treatment of patients with hemophilia A. BMN 270 is an AAV-factor VIII vector, designed to restore factor VIII plasma concentrations, essential for blood clotting in patients with hemophilia A. The gene therapy program for Hemophilia A was originally licensed from University College London and St. Jude Children's Research Hospital in February 2013 and has since been developed at BioMarin's facilities.
"Hemophilia A results from mutations at the genetic level, making gene therapy a potentially powerful technique to treat patients with a single dose," stated Hank Fuchs, M.D., Executive Vice President, Chief Medical Officer of BioMarin. "For the first clinical trial of BMN 270, we are looking to demonstrate that treatment with BMN 270 increases the expression of the factor VIII protein, necessary for blood clotting."
"The global bleeding disorders community greatly benefits from a wide range of support to help advance our vision of Treatment for All," said Alain Weill, World Federation of Hemophilia (WFH) President. "We welcome BioMarin as a new WFH Corporate Partner and greatly appreciate their commitment to support people with hemophilia A through their innovative gene therapy research."
Study Design
The Phase 1/2 study will evaluate the safety and efficacy of BMN 270 gene therapy in up to 12 patients with severe Hemophilia A. The primary endpoints are to assess the safety of a single intravenous administration of a recombinant AAV, human-coagulation Factor VIII vector and to determine the change from baseline of Factor VIII expression level at 16 weeks after infusion. The kinetics, duration and magnitude of AAV-mediated Factor VIII activity in individuals with hemophilia A will be determined and correlated to an appropriate BMN 270 dose. This is a dose escalation study with the goal of observing an increase in Factor VIII levels. Secondary endpoints include assessing the impact of BMN 270 on the frequency of Factor VIII replacement therapy, the number of bleeding episodes requiring treatment and any potential immune responses. Patients will be monitored for safety for five years.
Sunday, September 27, 2015
Dirk Hausseckar tweets Sangamo Related Info
$SGMO key competitive technological advantage: obligate heterodimers for impressive targeting specificity.0 retweets 1 favorite
Friday, September 25, 2015
Sangamo short Interest
Sangamo Short Interest (SGMO)
Sangamo Short Interest (SGMO)
DATE SHORT INTEREST
9/15/15 10,882,795
8/31/15 10,150,146
8/14/15 9,488,474
7/31/15 9,207,150
7/15/15 9,205,202
6/30/15 9,387,951
6/15/15 9,393,825
5/29/15 8,938,987
5/15/15 8,668,559
4/30/15 8,198,983
4/15/15 8,050,307
3/31/15 8,285,803
3/13/15 8,441,291
2/27/15 8,939,000
2/13/15 9,268,065
1/30/15 9,082,814
1/15/15 9,387,913
Days to cover as of 9/15/15 is 4.51
6/15/15 9,393,825
5/29/15 8,938,987
5/15/15 8,668,559
4/30/15 8,198,983
4/15/15 8,050,307
3/31/15 8,285,803
3/13/15 8,441,291
2/27/15 8,939,000
2/13/15 9,268,065
1/30/15 9,082,814
1/15/15 9,387,913
Days to cover as of 9/15/15 is 4.51
Thursday, September 24, 2015
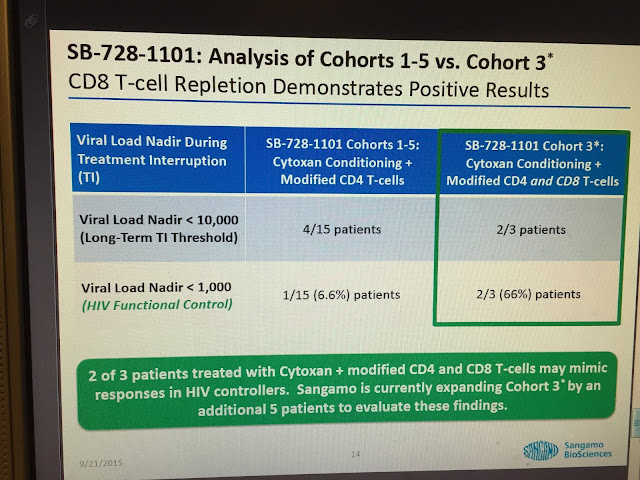
Aidsmeds.com Reports on Cohort 3
Gene Therapy Controls HIV Longer Than Four Months in Study
Cohort 3 includes three HIV-positive participants who received a genetic treatment in which their own CD4 and CD8 immune cells were drawn out, genetically modified to resist HIV, and then infused back into their bodies. This treatment was different from what was given to other participants in the study because it included modified CD8 cells instead of just modified CD4s. All of the study participants received a treatment called Cytoxan preconditioning before they received the modified cells in order to prime the body to better accept the immune cell infusion.
After undergoing the genetic treatment, the members of Cohort 3 all stopped taking ARVs. Two of them have maintained control of HIV for longer than 16 weeks.
“The ability of subjects treated in Cohort 3 to suppress and sustain control of viral load, combined with durable increases in CD4 and CD8 cells, provide support for our hypothesis of an immunologic mechanism of action for SB-728,” Dale Ando, MD, Sangamo’s vice president of therapeutic development and chief medical officer, said in a press release. “The prolonged positive effects observed in these Cohort 3 subjects have not been seen before with other treatments and have encouraged us to enroll and treat an additional five subjects with this regimen.”
To read a press release about the study, click here.
Tuesday, September 22, 2015
Monday, September 21, 2015
Sangamo's Press Release Today
Sunday, September 20, 2015
Urnov Presented Paper at ESGCT/FSGT 9/19/15
Efficient targeted gene addition to a safe harbor locus in long-term
repopulating hematopoietic stem cells for correction of X-linked
Chronic Granulomatous Disease via genome editing
Fyodor Urnov, Sangamo BioSciences Inc, Richmond CA
I do not have access to his presentation at ESGCT but this is the abstract from ASGCT May 2015:
54] Genome Editing of Primary Human CD34+ Hematopoietic Stem Cells Enables a Safe Harbor Targeted Gene Addition Therapeutic Strategy for Chronic Granulomatous Disease
Suk See De Ravin, Andreas Reik, Pei-Qi Liu, Linhong Li, Madhusudan V. Peshwa, Narda Theobald, Uimook Choi, Janet Lee, Sherry Koontz, Gary Lee, Philip D. Gregory, Fyodor D. Urnov, Harry L. Malech. Laboratory of Host Defenses, NIAID, NIH, Bethesda, MD; Sangamo BioSciences, Inc., Richmond, CA; MaxCyte, Inc, Gaithersburg, MD
Many monogenic recessive diseases of blood can, in principle, be cured by transfer of functional therapeutic transgene to the genome of the hematopoietic stem cell (HSC) – a strategy proven successful for multiple rare diseases using current integrating vector gene therapy. With a focus on X-linked chronic granulomatous disease (X-CGD), we report a directed approach orthogonal to randomly integrating retrovector gene therapy: the highly specific targeted placement of the curative transgene into a validated safe harbor locus in human HSCs via human genome editing with zinc finger nucleases (ZFNs) and donor insert delivery using an AAV6 vector.
We describe here an integrated targeted delivery platform customized for targeted addition to human HSCs using a cGMP-compliant electroporation system compatible with clinical scale production. Using next-generation, highly optimized ZFNs against the AAVS1/PPP1R12C gene locus, we optimized conditions for addition of the fluorescent Venus cDNA into human peripheral blood G-CSF mobilized CD34+ HSCs. Venus expression in manipulated human HSCs in vitro reached >50% efficiency, while earlier experiments demonstrated persistence of gene-modified cells in NSG mice with 12-15% Venus+ human CD45+ cells retrieved from transplanted mouse bone marrow and overall human HSC engraftment levels of >15%. Targeted integration (TI) rates achieved in human CD45+ cells from mouse bone marrow were 28-57%. Similar levels of Venus+ (~10%) are observed in spleen and peripheral blood CD45+ cells, indicating differentiation of gene-modified CD34 HSCs into circulating blood cells.
X-CGD patients suffer from severe bacterial and fungal infections with excessive inflammation due to a defect in the gp91phox subunit of phagocyte oxidase. To extend the results above to CGD, we therefore used the same approach to target addition of the relevant curative transgene, gp91phox, into the AAVS1 safe harbor locus of HSCs from patients with X-linked CGD. In vitro levels of gp91phox expression in gene-modified patient CD34+ HSCs population achieve 12-16% gp91phox expression by flow cytometric analysis, with an NSG xenograft study demonstrating 3-5% of the engrafted human CD45+ cells expressing gp91phox. Of note, the MND-driven gp91phox expression from the safe harbor locus in human neutrophils differentiating from CD34+ cells transplanted into the NSG mouse model parallels wildtype gp91phox levels produced at the native locus.
Our studies demonstrate the feasibility of targeted addition of different genes at the AAVS1 safe harbor site of the genome in human HSCs at an unprecedented efficiency and specificity; we demonstrate the efficient correction of the enzymatic defect in neutrophils arising from patient-derived HSCs in vivo. In sum with the advances in GMP-scale cell processing for genome editing, and the charted regulatory path for ZFNs to the clinic provided by ongoing trials in HIV, our studies represent the foundation for a rapid translation of ZFN-driven targeted addition as a clinical modality for X-linked CGD.
Keywords: Hematopoietic Stem Cells; Gene Correction/Modification/Targeting; Zinc-finger nucleases
repopulating hematopoietic stem cells for correction of X-linked
Chronic Granulomatous Disease via genome editing
Fyodor Urnov, Sangamo BioSciences Inc, Richmond CA
I do not have access to his presentation at ESGCT but this is the abstract from ASGCT May 2015:
54] Genome Editing of Primary Human CD34+ Hematopoietic Stem Cells Enables a Safe Harbor Targeted Gene Addition Therapeutic Strategy for Chronic Granulomatous Disease
Suk See De Ravin, Andreas Reik, Pei-Qi Liu, Linhong Li, Madhusudan V. Peshwa, Narda Theobald, Uimook Choi, Janet Lee, Sherry Koontz, Gary Lee, Philip D. Gregory, Fyodor D. Urnov, Harry L. Malech. Laboratory of Host Defenses, NIAID, NIH, Bethesda, MD; Sangamo BioSciences, Inc., Richmond, CA; MaxCyte, Inc, Gaithersburg, MD
Many monogenic recessive diseases of blood can, in principle, be cured by transfer of functional therapeutic transgene to the genome of the hematopoietic stem cell (HSC) – a strategy proven successful for multiple rare diseases using current integrating vector gene therapy. With a focus on X-linked chronic granulomatous disease (X-CGD), we report a directed approach orthogonal to randomly integrating retrovector gene therapy: the highly specific targeted placement of the curative transgene into a validated safe harbor locus in human HSCs via human genome editing with zinc finger nucleases (ZFNs) and donor insert delivery using an AAV6 vector.
We describe here an integrated targeted delivery platform customized for targeted addition to human HSCs using a cGMP-compliant electroporation system compatible with clinical scale production. Using next-generation, highly optimized ZFNs against the AAVS1/PPP1R12C gene locus, we optimized conditions for addition of the fluorescent Venus cDNA into human peripheral blood G-CSF mobilized CD34+ HSCs. Venus expression in manipulated human HSCs in vitro reached >50% efficiency, while earlier experiments demonstrated persistence of gene-modified cells in NSG mice with 12-15% Venus+ human CD45+ cells retrieved from transplanted mouse bone marrow and overall human HSC engraftment levels of >15%. Targeted integration (TI) rates achieved in human CD45+ cells from mouse bone marrow were 28-57%. Similar levels of Venus+ (~10%) are observed in spleen and peripheral blood CD45+ cells, indicating differentiation of gene-modified CD34 HSCs into circulating blood cells.
X-CGD patients suffer from severe bacterial and fungal infections with excessive inflammation due to a defect in the gp91phox subunit of phagocyte oxidase. To extend the results above to CGD, we therefore used the same approach to target addition of the relevant curative transgene, gp91phox, into the AAVS1 safe harbor locus of HSCs from patients with X-linked CGD. In vitro levels of gp91phox expression in gene-modified patient CD34+ HSCs population achieve 12-16% gp91phox expression by flow cytometric analysis, with an NSG xenograft study demonstrating 3-5% of the engrafted human CD45+ cells expressing gp91phox. Of note, the MND-driven gp91phox expression from the safe harbor locus in human neutrophils differentiating from CD34+ cells transplanted into the NSG mouse model parallels wildtype gp91phox levels produced at the native locus.
Our studies demonstrate the feasibility of targeted addition of different genes at the AAVS1 safe harbor site of the genome in human HSCs at an unprecedented efficiency and specificity; we demonstrate the efficient correction of the enzymatic defect in neutrophils arising from patient-derived HSCs in vivo. In sum with the advances in GMP-scale cell processing for genome editing, and the charted regulatory path for ZFNs to the clinic provided by ongoing trials in HIV, our studies represent the foundation for a rapid translation of ZFN-driven targeted addition as a clinical modality for X-linked CGD.
Keywords: Hematopoietic Stem Cells; Gene Correction/Modification/Targeting; Zinc-finger nucleases
Friday, September 18, 2015
Here is the Fetal Hemoglobin Paper EL mentioned in the Morgan Stanley Conference
Hemoglobin switching's surprise: the versatile transcription factor BCL11A is a master repressor of fetal hemoglobin.
Abstract
Copyright © 2015 Elsevier Ltd. All rights reserved.
Slippery Slope
The genetic manipulation of human IVF embryos is set to start in Britain for the first time following a licence application by scientists who want to understand why some women suffer repeated miscarriages.
If the research licence is granted by the Government’s fertility watchdog it will be only the second known occasion in the world where the chromosomes of human embryos have been genetically manipulated using a revolutionary gene-editing technique called Crispr/Cas9.
When Chinese scientists announced earlier this year that they had genetically altered “spare” human IVF embryos using Crispr/Cas9 for research purposes, there was deep concern among many who thought that they had gone too far – the US Government later imposed a moratorium on federally-funded research in America.
Read the article here:
http://www.independent.co.uk/news/science/ivf-embryos-to-be-genetically-manipulated-as-scientists-investigate-repeated-miscarriages-10506064.html
When Chinese scientists announced earlier this year that they had genetically altered “spare” human IVF embryos using Crispr/Cas9 for research purposes, there was deep concern among many who thought that they had gone too far – the US Government later imposed a moratorium on federally-funded research in America.
Read the article here:
http://www.independent.co.uk/news/science/ivf-embryos-to-be-genetically-manipulated-as-scientists-investigate-repeated-miscarriages-10506064.html
Wednesday, September 16, 2015
Japan Liberalizes Approval Process for Gene Therapy Treatments
Regenerative medicines in Japan can now get conditional marketing approval based on results from mid-stage, or Phase II, human trials that demonstrate safety and probable efficacy.Japan is liberalizing because with their aging population treatments for diseases like Alzheimer’s and Parkinson’s disease are in high demand.
Once lagging behind the United States and the European Union on approval times, there is now an approximately three-year trajectory for approvals, according to Frost’s Kumar. That compares with seven to 10 years before. …
Around the world, companies have also faced setbacks while pushing such treatments. In the U.S., Geron Corp., which started the first nation-approved trial of human embryonic stem cells, ended the program in 2011, citing research costs and regulatory complexities. …
While scientists globally have worked for years in this field, treatments have been slow to come to market. But there is hope in Japan that without the political red tape, promising therapies will emerge faster and there will be speedier rewards.
Under the new system, a firm with a gene or regenerative therapy (e.g. stem cells) can get conditional approval with a small trial. Conditional approval means that the firm will be able to sell its procedure while continuing to gather data on efficacy for a period of up to seven years. At the end of the seven year period, the firm must either apply for final marketing approval or withdraw the product.
http://fee.org/anythingpeaceful/japan-liberalizes-regenerative-medicine-and-gene-therapy/
Tuesday, September 15, 2015
Mitchell Finer, Former Bluebird Bio CSO Lands New Job
The predecessor of Phil Gregory as CSO of Bluebird Bio (BLUE) Mitchell Finer is now Managing Director of MPM Capital.
MPM’s Managing Director Todd Foley states: “The focus of our latest fund is to achieve breakthroughs that will cure previously incurable diseases. Accordingly, cell and gene therapy are extremely important areas of future opportunity for MPM, and Mitch is an acknowledged scientific leader in this space. Our portfolio companies will benefit greatly from his expertise, and we are privileged and tremendously grateful that Mitch has elected to share his considerable talents and experience with us and our portfolio.”
"The addition of Mitch to our team caps a momentous half-year of hiring for MPM in which we've added half a dozen new operating partners with expertise ranging from R&D (Mitch and Patrick Baeuerle) to IP (Greg Sieczkiewicz) to clinical development (Briggs Morrison and Pablo Cagnoni) to M&A and licensing (Tony Rosenberg)," said MPM co-founder Ansbert Gadicke.
For three decades, Mitch has focused on regenerative medicine, cancer immunotherapy and cell and gene therapy, helping to advance niche products from product conception through phase III clinical programs.
http://www.businesswire.com/news/home/20150915006799/en/MPM-Capital-appoints-Mitchell-H.-Finer-PhD#.VfjeH50o7vY
MPM’s Managing Director Todd Foley states: “The focus of our latest fund is to achieve breakthroughs that will cure previously incurable diseases. Accordingly, cell and gene therapy are extremely important areas of future opportunity for MPM, and Mitch is an acknowledged scientific leader in this space. Our portfolio companies will benefit greatly from his expertise, and we are privileged and tremendously grateful that Mitch has elected to share his considerable talents and experience with us and our portfolio.”
"The addition of Mitch to our team caps a momentous half-year of hiring for MPM in which we've added half a dozen new operating partners with expertise ranging from R&D (Mitch and Patrick Baeuerle) to IP (Greg Sieczkiewicz) to clinical development (Briggs Morrison and Pablo Cagnoni) to M&A and licensing (Tony Rosenberg)," said MPM co-founder Ansbert Gadicke.
For three decades, Mitch has focused on regenerative medicine, cancer immunotherapy and cell and gene therapy, helping to advance niche products from product conception through phase III clinical programs.
http://www.businesswire.com/news/home/20150915006799/en/MPM-Capital-appoints-Mitchell-H.-Finer-PhD#.VfjeH50o7vY
Dimension Therapeutics Files for IPO
From Firecebiotech.com:
Dimension Therapeutics is preparing to execute the next carefully planned step in its evolution, filing for a $115 million IPO close to 5 months after its crossover round landed. The filing came just days after the FDA accepted Cambridge, MA-based Dimension's IND for its lead gene therapy program, targeting a genetic fix for hemophilia B.
Dimension's lead program for DTX101 uses an AAV vector to deliver Factor IX into hemophilia patients. It's one of several biotechs to focus their first gene therapy programs on hemophilia, which a number of experts believe makes a logical focus for the new wave of upstarts now working in the field.
Gene therapy has been booming over the past two years as developers like bluebird bio ($BLUE) have powered their way back in after the first generation of work was shelved following the emergence of lethal side effects. Biogen ($BIIB) focused its new gene therapy group on hemophilia, alongside Spark Therapeutics ($ONCE), RegenX--which outlicensed original tech to Dimension--and others. But for now there's more promise than performance, as new data start to reveal whether a single-dose fix can work safely.
http://www.fiercebiotech.com/story/gene-therapy-player-dimension-catches-biotech-ipo-wave-files-115m-offering/2015-09-14
Dimension Therapeutics is preparing to execute the next carefully planned step in its evolution, filing for a $115 million IPO close to 5 months after its crossover round landed. The filing came just days after the FDA accepted Cambridge, MA-based Dimension's IND for its lead gene therapy program, targeting a genetic fix for hemophilia B.
Dimension's lead program for DTX101 uses an AAV vector to deliver Factor IX into hemophilia patients. It's one of several biotechs to focus their first gene therapy programs on hemophilia, which a number of experts believe makes a logical focus for the new wave of upstarts now working in the field.
Gene therapy has been booming over the past two years as developers like bluebird bio ($BLUE) have powered their way back in after the first generation of work was shelved following the emergence of lethal side effects. Biogen ($BIIB) focused its new gene therapy group on hemophilia, alongside Spark Therapeutics ($ONCE), RegenX--which outlicensed original tech to Dimension--and others. But for now there's more promise than performance, as new data start to reveal whether a single-dose fix can work safely.
http://www.fiercebiotech.com/story/gene-therapy-player-dimension-catches-biotech-ipo-wave-files-115m-offering/2015-09-14
Thursday, September 10, 2015
First Application of Sangamo's In Vivo Protein Replacement Platform (IVPRP) for Potential Cure of Hemophilia B
From today's press release:
"Hemophilia B is the first clinical application of our IVPRP which can be applied to many other diseases that are currently treated by protein replacement including hemophilia A and lysosomal storage disorders," saidGeoff Nichol , M.B., Ch.B., Sangamo's executive vice president of research and development. "Successful review, by the NIH RAC, of the first human in vivo genome editing clinical protocol is a major milestone for Sangamo. We appreciate this careful consideration and unanimous approval of our proposed clinical trial and look forward to the commencement of our Phase 1 study in the near future."
IMPRESSIVE WORK !!
"Hemophilia B is the first clinical application of our IVPRP which can be applied to many other diseases that are currently treated by protein replacement including hemophilia A and lysosomal storage disorders," said
IMPRESSIVE WORK !!
Wednesday, September 9, 2015
Sangamo BioSciences Announces Presentation At The 2015 Morgan Stanley Global Healthcare Conference
9/9/15 RAC Approves Sangamo Hemophilia F9 Protocol
Tip of the hat to Dr. Holmes.
RAC comments "exciting and innovative and very sophisticated protocol"
All votes YES
RAC comments "exciting and innovative and very sophisticated protocol"
All votes YES
Tuesday, September 8, 2015
Interesting Tweets from @RNAiAnalyst
Read from bottom
$SGMO, however, has IP advantage: 1st mover in a number of Rx areas; plus in ZFN no stepping over each others' toes.0 retweets 0 favorites
- What stuck out is that ZFN/TALEN can do what CRISPR can, but gene k.o. cell line $30-40k vs 1k. does this matter for Rx development?
$SGMO0 retweets 1 favorite
- Nice market overview of gene editing in CEN: http://cen.acs.org/articles/93/i35/Genome-Editing-Writ-Large.html …
$SGMO
Reminder - Sangamo Presents F9 Data at RAC meeting Tomorrow 10 am est (SGMO)
Webcast can be found here:
You will be able to view the event at http://videocast.nih.gov when the event is live
You will be able to view the event at http://videocast.nih.gov when the event is live
Saturday, September 5, 2015
Sangamo Biosciences (SGMO) v. RA Capital Final Order and Judgement
As reported by Blackwatch on www.investorvillage.com a settlement has been reached in the RA Capital lawsuit. The document I have access to does not provide an amount but verifies that Sangamo has proved its claims as written in the complaint.
Sangamo Biosciences, Inc. v. RA Capital Healthcare Fund, L.P. et al
Court New York Southern District Court
Judge Paul A Crotty
Nature of Suit 850 Other Statutes - Securities/Commodities/Exchange
Cause 15:78m(a) Securities Exchange Act
Case # 1:15-cv-03004
Filed Apr 17, 2015
Terminated Aug 20, 2015
Sangamo Biosciences, Inc. v. RA Capital Healthcare Fund, L.P. et al
Court New York Southern District Court
Judge Paul A Crotty
Nature of Suit 850 Other Statutes - Securities/Commodities/Exchange
Cause 15:78m(a) Securities Exchange Act
Case # 1:15-cv-03004
Filed Apr 17, 2015
Terminated Aug 20, 2015
Friday, September 4, 2015
Review of Current Gene Therapy Market - Snngamo Mention (SGMO)
Pharmavoice.com has a new online review of the gene therapy market.
Here is the relevant Sangamo excerpt:
Here is the relevant Sangamo excerpt:
Biogen has a collaboration with Sangamo BioSciences in the area of zinc finger nucleases related to hemoglobinopathies, targeting sickle cell disease and beta-thalassemia. The beta-thalassemia program was initiated with a BCL11a knockout strategy, and the sickle cell program already employs the BCL11A enhancer approach.
Both beta-thalassemia and sickle cell manifest several months after birth, when patients’ cells switch from producing functional fetal globin to a mutant form of adult beta-globin, which causes their condition. The development program uses ZFN-mediated genome editing of a patient’s own hematopoietic stem and progenitor cells (HSPCs) to increase production of fetal globin in cells that will ultimately become red blood cells.
Sangamo and Biogen have developed two related but distinct ZFN-mediated genome editing approaches to disrupt critical aspects of the regulatory pathway that, in early infancy, lead to the switch in production from fetal to adult globin.
Initially, the companies developed a strategy for beta-thalassemia that specifically knocked out the gene encoding for the BCL11A transcription factor, a critical regulator of the switch from fetal to adult globin production.
A second approach was initiated for the sickle cell program, which involved the disruption of the more recently described erythroid-specific enhancer of BCL11A expression, a regulatory DNA sequence in the genome that is essential for expression of BCL11A but that is functional exclusively in cells destined to become RBCs.
Dr. Danos says clinical trials for both programs are expected to begin in 2016.
Read the entire article here:
http://www.pharmavoice.com/article/2015-09-gene-therapy/
Tuesday, September 1, 2015
Extraordinary Volume Today
Huge volume at the close; there was a trade of 1,162,200 .
Total volume for the day 2,784,369
Average volume 1,100,000
Volume precedes price?????
Total volume for the day 2,784,369
Average volume 1,100,000
Volume precedes price?????
Subscribe to:
Comments (Atom)
 Dirk Haussecker
Dirk Haussecker